Abstract
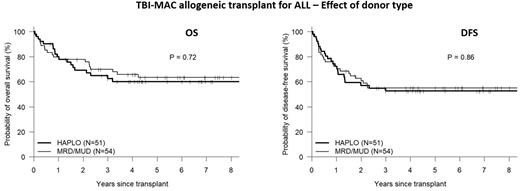
Data from the CIMBTR has previously demonstrated the non-inferiority of transplantation from a haploidentical donor (HAPLO) using post-transplant cyclophosphamide (PTCy) vs. matched related (MRD) or unrelated donors (MUD) for acute lymphoblastic leukemia (ALL) (Wieduwilt et al. Blood Advances 2022). However, total body irradiation (TBI)-containing myeloablative conditioning (MAC) was used in less than 60% of MRD/MUD patients and in approximately 40% of HAPLO patients. We aimed to better define the role of donor type (HAPLO vs. MRD/MUD) following TBI-MAC-based allogenic transplant for ALL. To this end, we analyzed 105 consecutive ALL patients receiving a first allogeneic transplant at our institution following TBI-MAC from an MRD [30], MUD [24] or HAPLO [51] donor between Jan 2011 to Sept 2021. HAPLO patients received a preparative regimen of fludarabine (90mg/m2) and TBI 1200 cGy, followed by post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate mofetil. MRD/MUD patients received TBI 1200 cGy with either Cy (120mg/kg, n= 33) or etoposide (60mg/kg, n=21), followed by tacrolimus and methotrexate for GVHD prophylaxis. Baseline characteristics include a median age of 40 (range, 29-51) years, HCT-CI ≥3 in 53%, poor risk cytogenetics in 60% (Ph+ [37%], Ph-like [11%], complex karyotype [7%] and 11q23 translocation [5%]). PBSC was the graft source in 94%. Pre-transplant status was CR1, CR2/3, or PIF/relapse in 75%, 12% and 13% respectively. Pre-transplant measurable residual disease (MRD) was seen in 28%). Baseline demographics of HAPLO and MRD/MUD recipients were similar with the exception of race/ethnicity, where HAPLO recipients (vs. MRD/MUD) were significantly less likely to be non-Hispanic White (29% vs. 67%). Comparison of baseline disease characteristics revealed more T-cell lineage disease (27% vs. 4%) and less poor risk cytogenetics (47% vs. 72%) in HAPLO vs. MRD/MUD recipients, respectively. After a median follow-up of 70 months (range, 8-134), 5-yr overall survival (OS), disease-free survival (DFS), cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) for the whole cohort was 62%, 54%, 29% and 18% respectively, with no significant differences between HAPLO and MRD/MUD transplants (see figure). In multivariable analysis, controlling for other significant covariates (race, pre-transplant disease status and pre-transplant MRD), donor type (HAPLO vs. MRD/MUD) did not have a significant impact on OS (HR 1.04, p=0.91)) or DFS (HR 0.99, p=0.97)). TBI-MAC-based HAPLO represents a favorable alternative donor source with comparable long-term DFS and OS. This is of particular importance to patients of racial/ethnic minorities, whereby rapid and practically universal access to a HAPLO donor allows for curative-intent therapy in the vast majority of high-risk ALL patients.
Disclosures
Solh:ADC Therapeutics: Research Funding; Partner Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.